This laboratory explores diffusion and osmosis, fundamental biological processes, utilizing dialysis tubing as a model membrane to observe molecule passage and membrane permeability;
Experiments with starch, glucose, and red onion cells demonstrate how solutions and molecular weight influence diffusion rates across semi-permeable membranes.
Analyzing results, including iodine tests and osmosis observations, provides insights into membrane selectivity and its relevance to living cells, aiding answer key comprehension.
Overview of the Experiment
This experiment meticulously investigates the principles of diffusion and osmosis, core concepts in understanding cellular transport mechanisms. Students will utilize dialysis tubing, functioning as a selectively permeable membrane, to observe the movement of various solutes – specifically glucose and starch – across its barrier.
The initial phase focuses on observing Brownian motion and qualitatively assessing diffusion. Subsequently, the experiment transitions to examining osmosis using red onion cells immersed in solutions of differing salt concentrations. Iodine serves as a crucial indicator to visually detect the presence, or absence, of starch.
Data collected will be analyzed to determine which molecules can permeate the membrane and how concentration gradients influence the rate of transport. The ultimate goal is to solidify comprehension of membrane permeability and its biological significance, preparing students to accurately interpret an answer key;
Importance of Understanding Membrane Permeability
Understanding membrane permeability is fundamental to grasping life’s processes, as cell membranes regulate the internal environment, controlling what enters and exits. This control is vital for nutrient uptake, waste removal, and maintaining cellular homeostasis. The diffusion lab directly illustrates these principles.
Knowledge of membrane function is crucial in fields like medicine, explaining drug delivery, kidney function, and nerve impulse transmission. Analyzing glucose and starch movement highlights how molecular weight impacts passage. Observing osmosis in onion cells demonstrates water potential’s role.
Successfully interpreting the lab’s results, and therefore the answer key, builds a foundation for understanding more complex biological systems and the consequences of disrupted membrane function, like cellular swelling or shrinking.

Materials and Methods
This experiment utilized dialysis tubing, glucose, starch, distilled water, iodine, red onion cells, salt solutions, microscopes, and beakers for observing diffusion and osmosis.
Dialysis Tubing as a Model Membrane
Dialysis tubing serves as a crucial model for biological membranes in this experiment, effectively mimicking their semi-permeable characteristics. Constructed from cellulose, it possesses tiny pores allowing smaller molecules, like glucose, to pass through while restricting larger molecules, such as starch.
This selective permeability is fundamental to understanding how cell membranes regulate substance transport. By observing diffusion across the tubing, we gain insight into the principles governing molecule movement across biological barriers. The tubing’s pore size directly influences which substances can permeate, mirroring the selective nature of cellular membranes.
Its use simplifies the study of diffusion and osmosis, providing a controlled environment for observing these processes and interpreting results related to membrane permeability.
Solutions Used in the Experiment
This experiment utilizes several key solutions to demonstrate diffusion and osmosis. Distilled water serves as the baseline, providing a solvent for observing solute movement. A starch solution, created by dissolving starch in water, represents a larger molecule tested for membrane permeability.
A glucose solution, similarly prepared, acts as a smaller molecule for comparison. Varying concentrations of salt solutions are employed in the osmosis portion, using red onion cells, to observe water movement across cell membranes.
These solutions, carefully prepared, allow for controlled observation of how different substances interact with the semi-permeable membrane, aiding in understanding diffusion principles and interpreting results.
Preparation of Solutions (Glucose, Starch, Distilled Water)
Accurate solution preparation is crucial for reliable diffusion and osmosis results. Distilled water is used as the solvent for all solutions, ensuring purity. The starch solution is made by carefully dissolving a measured amount of starch in warm distilled water, stirring until fully dissolved.
The glucose solution is prepared similarly, dissolving a specific mass of glucose in distilled water. Precise measurements are vital for quantitative analysis. Consistent stirring ensures complete dissolution, creating homogenous solutions.
Proper labeling of each solution prevents confusion, and maintaining consistent volumes is essential for comparative analysis during the experiment.
Setting Up the Diffusion Experiment
Dialysis tubing, representing the semi-permeable membrane, is soaked in distilled water to increase flexibility. Cut equal lengths of tubing and securely tie off one end of each piece. Fill each tube with either the starch solution or the glucose solution, avoiding air bubbles.
Carefully tie off the open ends of the tubing, creating sealed compartments. Place each filled tube into a beaker containing distilled water. Ensure the water level covers the tubing completely.
Label each beaker clearly, indicating the contents of the dialysis tubing. This setup allows observation of diffusion over time, facilitating data collection and analysis.

Observing Diffusion
Initial observations reveal clear solutions; monitoring time course shows changes as molecules move. Iodine detects starch passage, indicating membrane permeability differences.
Initial Observations of the Solutions
Before initiating the experiment, carefully document the initial appearance of each solution. The distilled water should be perfectly clear and colorless, serving as the control. The glucose solution will also appear clear, though potentially with a slight viscosity depending on concentration.
Notably, the starch solution will be cloudy or opaque due to the suspended starch particles. Inside the dialysis tubing, observe the initial color and clarity of the contained solution. Record any visible differences between the inside and outside of the tubing.
These baseline observations are crucial for accurately assessing changes during diffusion and interpreting the results later, especially when using indicators like iodine.
Time Course of Diffusion – Monitoring Changes
Throughout the experiment, observe the solutions at regular intervals – for example, every 15 or 30 minutes – and meticulously record any alterations in appearance. Pay close attention to the clarity of the distilled water surrounding the dialysis tubing.
A key indicator of glucose diffusion is the lack of visible change, as it’s colorless. However, monitor for any subtle shifts. For starch, observe if the cloudiness diminishes within the tubing, suggesting diffusion.
Document these changes qualitatively, noting the intensity of color or clarity. Consistent monitoring is vital for understanding the rate of diffusion and accurately interpreting the results.
Using Indicators to Detect Molecule Passage (Iodine for Starch)
Iodine solution serves as a crucial indicator for detecting the presence of starch. Add a few drops of iodine to the distilled water surrounding the dialysis tubing at regular intervals. A dark blue-black color change signifies starch has diffused across the membrane.
If no color change occurs, it indicates starch molecules haven’t passed through, confirming the semi-permeable membrane’s selectivity. This visual cue is essential for confirming diffusion or lack thereof.
Carefully observe and record the timing and intensity of any color changes, as this data is vital for analyzing the experiment’s results and answering related lab questions.

Osmosis and Cell Membranes
Osmosis, water diffusion across a membrane, is explored using red onion cells in varying salt solutions, demonstrating cellular responses to differing solute concentrations.
Osmosis: The Diffusion of Water
Osmosis is a specific type of diffusion focusing on the movement of water molecules across a semi-permeable membrane. This movement occurs from an area of high water concentration to an area of low water concentration, aiming to equalize solute levels on both sides.
Unlike general diffusion, osmosis requires a selectively permeable barrier. The lab utilizes this principle by observing how water moves into or out of cells, impacting their turgor pressure. Understanding osmosis is crucial as it governs water balance in biological systems.
The experiment demonstrates this with red onion cells, showcasing plasmolysis (cell shrinkage) in hypertonic solutions and potential lysis (cell bursting) in hypotonic solutions, providing key answer key insights.
Red Onion Cells and Osmosis
Red onion cells serve as an excellent model for observing osmosis due to their readily visible cell walls and central vacuoles. Placing these cells in varying salt solutions allows for clear visualization of water movement and its effect on cell structure.
In hypotonic solutions (low solute concentration), water enters the cells, causing them to swell and potentially burst. Conversely, hypertonic solutions (high solute concentration) draw water out, leading to plasmolysis – the cell membrane detaching from the cell wall.
Observing these changes under a microscope provides direct evidence of osmosis and aids in understanding the answer key concepts of water potential and cellular equilibrium.
Varying Salt Solutions and Their Effect on Onion Cells
Utilizing different salt concentrations – hypotonic, hypertonic, and isotonic – demonstrates the principles of osmosis in red onion cells. Hypotonic solutions (e.g., distilled water) cause water to rush into the cells, inflating the vacuoles and making the cells turgid.
Hypertonic solutions (e.g., high salt concentration) induce water to exit the cells, resulting in plasmolysis – the cell membrane pulls away from the rigid cell wall. Isotonic solutions maintain equilibrium, with no net water movement.
Careful observation under a microscope, coupled with the answer key, reveals these effects, illustrating how solute concentration impacts water potential and cellular integrity.
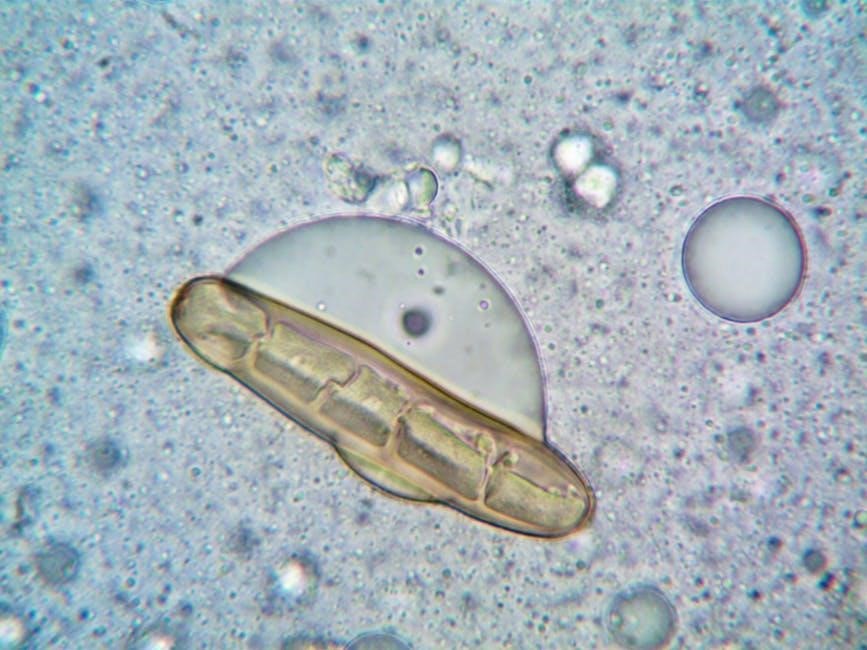
Analyzing Results
Examining starch and glucose diffusion, alongside osmosis in onion cells, allows for interpretation of membrane permeability and solution effects, using the answer key.
Starch Diffusion Results and Interpretation
Observations revealed that starch, a large polysaccharide, did not diffuse across the dialysis tubing, the model membrane, even after a significant time period. The iodine test, used as an indicator, remained negative outside the tubing, confirming starch retention within.
This result demonstrates that membrane pore size restricts the passage of larger molecules like starch. The answer key confirms this, highlighting membrane selectivity based on molecular weight. Diffusion is hindered due to the size disparity.
Conversely, the solution inside the tubing remained consistently positive for starch, indicating no leakage. This supports the understanding of semi-permeable membrane characteristics and their role in regulating molecular transport.
Glucose Diffusion Results and Interpretation
Glucose, a smaller monosaccharide, did diffuse across the dialysis tubing membrane over time. While a direct visual indicator wasn’t used, the answer key anticipates a gradual decrease in glucose concentration inside the tubing and a corresponding increase outside.
This result illustrates that smaller molecules can readily pass through the membrane’s pores via diffusion, driven by the concentration gradient. The rate of diffusion is influenced by factors like temperature and concentration, as expected.
The experiment confirms that membrane permeability isn’t absolute; it’s selective, favoring smaller molecules like glucose over larger ones like starch, demonstrating a key principle of cellular transport.
Observations of Brownian Motion
Brownian motion, observed using a microscope with carmine red particles in water, demonstrates the random, erratic movement of particles. This movement isn’t due to any external force, but rather the collision of water molecules with the carmine particles – a key concept in understanding diffusion.
The answer key emphasizes that this random motion provides visual evidence for the kinetic energy of molecules. Observing this chaotic movement helps illustrate how molecules spread out from areas of high concentration to low concentration.
This initial observation sets the stage for understanding diffusion across a membrane, as it highlights the fundamental principle of molecular movement driving the process.

Understanding Membrane Selectivity
Membrane selectivity dictates which molecules pass through, based on size and properties. Diffusion rates are affected by molecular weight and membrane characteristics, as shown in the answer key.
Semi-Permeable Membrane Characteristics
Dialysis tubing, utilized in this experiment, functions as a model semi-permeable membrane. Its porous structure allows smaller molecules, like glucose, to pass through, while larger molecules, such as starch, are restricted. This selective permeability is crucial for understanding cellular processes.
The membrane’s characteristics directly impact diffusion rates; pore size and material composition determine which substances can traverse it. Observing the results – specifically, the absence of starch outside the tubing – confirms its selective nature. The answer key emphasizes this distinction, highlighting the membrane’s role as a barrier based on molecular weight.
Understanding these properties is fundamental to grasping how cells regulate transport and maintain internal homeostasis, mirroring real-world biological membranes.
Factors Affecting Diffusion Rate
Several factors influence the speed at which molecules move across a semi-permeable membrane during diffusion. Molecular weight is paramount; smaller molecules, like glucose, diffuse more rapidly than larger ones, such as starch, as observed in the experiment.
Concentration gradients also play a vital role – a steeper gradient accelerates diffusion. Temperature impacts kinetic energy, increasing diffusion rates with higher temperatures. The answer key stresses these relationships, explaining how these variables affect molecule passage.
Membrane permeability, determined by pore size and composition, further modulates the rate, impacting the results and overall understanding of transport mechanisms.
Molecular Weight and Membrane Permeability
The diffusion lab clearly demonstrates the inverse relationship between molecular weight and membrane permeability. Larger molecules, like starch polysaccharides, struggle to traverse the pores of the dialysis tubing – our model membrane – resulting in minimal diffusion.
Conversely, smaller molecules, such as glucose, readily pass through, showcasing higher permeability. The answer key emphasizes this, explaining how pore size acts as a selective barrier. This selectivity mirrors biological membranes, controlling substance transport.
Understanding this principle is crucial for interpreting results and appreciating how membrane properties dictate cellular function and diffusion rates.

Lab Report Components
Reports require detailed data collection, clear calculations, and a thorough discussion of results, including potential error analysis, referencing the answer key.
Data Collection and Presentation
Meticulous data recording is crucial; document initial and subsequent observations of solution color changes, noting any diffusion evidence like iodine reactions with starch.
Quantitative data should include time intervals and qualitative assessments of color intensity, presented in organized tables and graphs for clarity.
Osmosis observations of red onion cells require detailed descriptions of cell shape changes in varying salt solutions, ideally with microscopic images.
Brownian motion observations should include sketches or descriptions of particle movement. Ensure all data directly supports answer key interpretations and conclusions.
Proper labeling of axes and clear captions are essential for effective presentation.
Calculations and Quantitative Analysis
Quantitative analysis involves calculating diffusion rates based on observed changes in solution color or starch/glucose presence, using iodine test results as indicators.
For osmosis, determine percentage changes in red onion cell size in different salt solutions, reflecting water movement across the membrane.
Data from tables can be graphed to visualize diffusion or osmosis rates over time, aiding in trend identification.
Relate calculations to molecular weight; larger molecules like starch exhibit slower diffusion.
These analyses provide numerical support for conclusions and validate the answer key’s expected outcomes.
Discussion of Results and Error Analysis
Results should confirm starch’s inability to cross the membrane, indicated by a persistent blue/purple color with iodine, while glucose diffuses, aligning with the answer key.
Osmosis in red onion cells demonstrates plasmolysis in hypertonic solutions and swelling in hypotonic ones, validating water potential principles.
Error analysis should address potential issues like temperature fluctuations, imprecise measurements, or incomplete mixing of solutions.
Discuss how these errors might have affected diffusion/osmosis rates and the accuracy of conclusions.
Relate findings to real-world biological systems and acknowledge limitations of the dialysis tubing model.
Answer Key Considerations
Expected results show starch remains within the membrane, while glucose diffuses; onion cells exhibit plasmolysis/turgor based on solution salinity.
Key questions address diffusion principles and osmosis’s impact on cell structure, aligning with the lab’s objectives.
Expected Results for Starch and Glucose Diffusion
Initially, both starch and glucose should be present within the dialysis tubing. However, over time, a distinct difference will emerge due to varying molecular sizes and membrane permeability.
Glucose, being a smaller molecule, is expected to diffuse across the semi-permeable membrane into the surrounding distilled water, resulting in a positive test for glucose outside the tubing.
Conversely, starch, a much larger polysaccharide, should largely remain confined within the dialysis tubing, indicated by a continued positive iodine test inside, but a negative test outside.

Indicators like iodine confirm this, turning dark blue/purple in the presence of starch. This demonstrates the membrane’s selective permeability, allowing smaller molecules to pass while restricting larger ones.
Interpreting Osmosis Results in Onion Cells
Red onion cells immersed in varying salt solutions will exhibit clear osmotic changes. In hypotonic solutions (low salt), water will rush into the cells, causing them to swell and potentially burst, appearing turgid under a microscope.
Conversely, in hypertonic solutions (high salt), water will move out of the cells, leading to plasmolysis – the cell membrane pulling away from the cell wall, resulting in a shriveled appearance.
Isotonic solutions (equal salt concentration) will maintain cell equilibrium, with no net water movement, and cells will appear normal. Observing these changes allows interpretation of water potential and membrane permeability.
Addressing Common Lab Report Questions
Common questions often ask why starch didn’t diffuse through the membrane – its large molecular weight prevents passage. Explaining why glucose did diffuse requires understanding its smaller size and ability to cross the semi-permeable membrane.
Regarding osmosis, students should connect salt concentration to water movement, explaining plasmolysis in hypertonic solutions and turgidity in hypotonic ones. Error analysis should address potential inaccuracies in solution preparation or observation.
Discussing Brownian motion demonstrates the random movement of molecules driving diffusion. Properly interpreting these phenomena is key to a successful lab report.

Further Exploration
Diffusion and osmosis are vital in biological systems like nutrient absorption and waste removal; explore how these principles apply to kidney function and plant biology.
Real-World Applications of Diffusion and Osmosis
Diffusion and osmosis aren’t confined to the lab; they are essential processes underpinning numerous real-world phenomena. Consider medical applications like dialysis, where diffusion removes waste products from the blood when kidneys fail, mimicking membrane function.
In agriculture, osmosis explains water uptake by plant roots from the soil. Food preservation techniques, such as salting or sugaring, utilize osmosis to draw water out of microorganisms, inhibiting their growth.
Furthermore, understanding these principles is crucial in developing effective drug delivery systems, ensuring medications reach target tissues efficiently. Even everyday occurrences, like a sponge absorbing water, demonstrate osmosis in action, highlighting its pervasive influence.
Connecting Lab Results to Biological Systems
Our lab findings directly mirror processes within living organisms. The dialysis tubing serves as an analog for cell membranes, demonstrating selective permeability – allowing small molecules like glucose to pass while restricting larger ones like starch.
This parallels how nutrient absorption occurs in the small intestine, where small molecules diffuse into the bloodstream. The osmosis observed with red onion cells reflects water balance in plant and animal cells, crucial for maintaining turgor pressure and cell volume.
Understanding these diffusion principles is fundamental to comprehending cellular transport, signaling, and overall physiological function, bridging the gap between experimental observation and biological reality.
